Salt Water May Periodically Form on the Surface of Mars
Briny water may form on the surface of Mars a few days per year, research by Planetary Science Institute Senior Scientist Norbert Schorghofer shows.
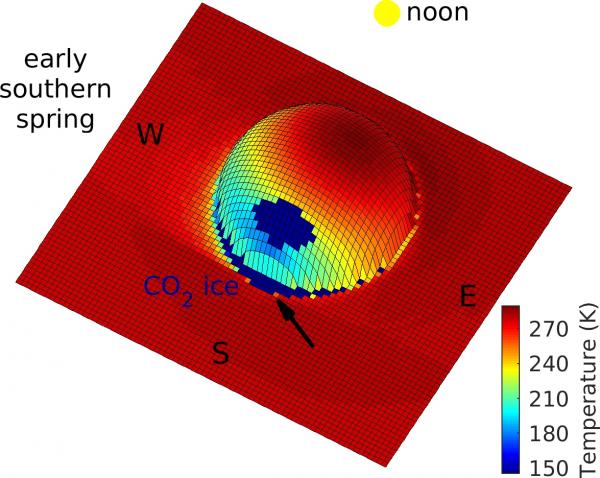
Three-dimensional view of the Martian surface temperature around an idealized boulder at latitude 30°S. On the side opposite to the Sun, temperatures are around -128° Celsius, and as the sun rises, this area heats up rapidly, so frost melts on salt-containing ground before it sublimates into the atmosphere.
Liquid water is difficult to come by on Mars, because ice rapidly dissipates, or sublimates, into the atmosphere long before it reaches its melting point. That is because the atmospheric pressure on Mars lies near the triple point pressure of H2O, the minimum pressure necessary for liquid water to exist.
“Mars has plenty of cold ice-rich regions and plenty of warm ice-free regions, but icy regions where the temperature rises above the melting point are a sweet spot that is nearly impossible to find. That sweet spot is where liquid water would form,” Schorghofer said.
The process works as follows: A boulder sitting on the surface at mid-latitudes casts a shadow in winter. The continually shadowed area behind the boulder is very cold, so cold that water ice accumulates in winter. When the Sun rises again in spring, the ice suddenly heats up. In detailed model calculations, the temperature rises from -128° Celsius in the morning to -10° Celsius at noon, a huge change over a quarter of a day. Over such a short time, not all of the frost is lost to the atmosphere.
Salt depresses the melting point of H2O, so on salt-rich ground, water ice will melt at -10° Celsius. Brines, or salty water, will form until all of the ice has either turned to liquid or vapor. Next Mars year, the same process repeats.
The shadowed areas behind the boulders are so cold in winter that not only water frost but also carbon dioxide ice builds up. For Mars, the first day without carbon dioxide ice in spring is called the "crocus date." Melting occurs on or immediately after the crocus date, and therefore the term “crocus melting”.
“Answering the question whether crocus melting of seasonal water ice actually occurs on Mars required a slew of detailed quantitative calculations — the numbers really matter,” Schorghofer said. “It took decades to develop the necessary quantitative models.”
Source: Planetary Science Institute
- 297 reads
Human Rights
Ringing FOWPAL’s Peace Bell for the World:Nobel Peace Prize Laureates’ Visions and Actions

Protecting the World’s Cultural Diversity for a Sustainable Future

The Peace Bell Resonates at the 27th Eurasian Economic Summit

Declaration of World Day of the Power of Hope Endorsed by People in 158 Nations

Puppet Show I International Friendship Day 2020

