Monoclonal antibodies against Zika show promise in monkey study
Further development toward clinical evaluation is warranted.
Using blood samples from an individual previously infected with Zika virus, scientists funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, have developed an antibody-based Zika virus therapeutic that protected monkeys from infection. Because monoclonal antibodies are generally safe, they believe that this antibody cocktail might be appropriate for uninfected pregnant women; because the antibodies will likely cross the placenta, the researchers hope that administration during pregnancy may protect both the pregnant woman and the fetus from Zika virus. The investigators are hoping to test this concept by pursuing studies in people.
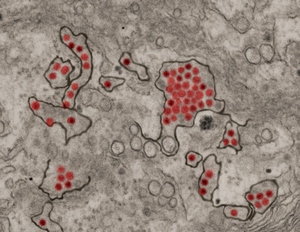
Zika virus particles (red) shown in African green monkey kidney cells.
The scientists isolated immune cells from the patient’s blood and used them to make 91 monoclonal antibodies—immune system fighters designed to bind to a specific part of an invading virus or bacterium to stop the infection. They identified three antibodies that bound to Zika virus surface proteins, and each neutralized the virus. The researchers then administered a combination of these antibodies to rhesus macaques and exposed the animals to Zika virus one day later. During the 21-day study, all four monkeys who received the antibody cocktail showed no virus replication.
Researchers at the University of Miami and The Scripps Research Institute led the project with collaborators in Brazil and the U.S.
Source: U.S. National Institutes of Health
- 310 reads
Human Rights
Ringing FOWPAL’s Peace Bell for the World:Nobel Peace Prize Laureates’ Visions and Actions

Protecting the World’s Cultural Diversity for a Sustainable Future

The Peace Bell Resonates at the 27th Eurasian Economic Summit

Declaration of World Day of the Power of Hope Endorsed by People in 158 Nations

Puppet Show I International Friendship Day 2020

